To keep the math simple, we will ignore the three-dimensional nature of the cell. In reality, we would be interested, for example, in how long it would a molecule to diffuse in three dimensions from the edge of the cell (the cell membrane) to somewhere in the center of the cell. Instead, we'll model one-dimensional diffusion across an interval.
Let's imagine that the molecule is diffusing from the edge of the cell to some location a distance $L$ away. When the molecule reaches that location, it is immediately removed (for example, it could be metabolized into another product). In the one-dimensional model, we'll let $x=0$ represent the edge of the cell and $x=L$ represent the final location near the center of the cell. (The length $L$ could represent the radius of the cell.) We are interested in how long it would take a molecule to diffuse from $x=0$ to $x=L$.
Rather than rely on simulations, we will develop a mathematical model of the diffusion of the molecules along this interval. Let $c(x,t)$ be the concentration (measured in molecules per mm) of the molecules at position $x$ (measured in mm) at time $t$ (measured in seconds). Our goal is to develop an equation for the dynamics of $c$.
We will imagine that the concentration of molecules outside the cell is at some fixed level. Let $c_0$ be this external concentration. Since the outside space represents a huge reservoir, we'll imagine that, even as molecules diffuse into the cell, the external concentration remains unchanged.
The condition that the concentration at the edge of the cell is fixed at $c_0$ is a boundary condition. Write the equation for this boundary condition in terms of $c(x,t)$. (Hint: in the boundary condition, one of the arguments of $c$ should be fixed to a number, but the other argument should be left as is.)
Inside the interval $x \in (0,L)$, we want an equation for the rate of change of the concentration, i.e., the derivative with respect to time $\pdiff{c}{t}$. (We use the partial derivative symbol because $c(x,t)$ is a function of both $x$ and $t$.)
The concentration $c(x,t)$ will change based on the flux of ions across any point $x$. Denote this flux by $J(x,t)$. $J(x,t)$ is simply the net number of molecules per second crossing $x$ from the left. It is the “net” number, as molecules are jumping rightward across $x$ (crossing $x$ from the left) as well as jumping leftward across $x$ (crossing $x$ from the right). A leftward jump across $x$ cancels out a rightward jumps across $x$ (as far as the concentration is concerned), so we only care if there are more rightward jumps across $x$ (in which case $J(x,t)$ would be positive) or more leftward jumps across $x$ (in which case $J(x,t)$ would be negative).
For now, let's not worry about a formula for $J(x,t)$. We'll figure that out later based on how the molecules jump left and right. For now, we want a formula for the change in concentration $\pdiff{c}{t}(x,t)$ as a function of the flux $J(x,t)$.
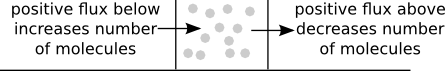
Let's think about how the number of molecules in an small region around $x$ could change, as illustrated in the above diagram. The number of molecules in that region will increase if there is a positive flux $J$ just below $x$ (since that flux represents molecules arriving from below) and the number of molecules in that region will decrease if there is a positive flux $J$ just above $x$ (since that flux represents molecules leaving to go to larger values of $x$). Whether the number of molecules around $x$ is increasing or decreasing depends on whether the flux below or above is larger.
The number of molecules at time $t$ will be proportional to the concentration at the point, $c(x,t)$. We can capture how this concentration $c(x,t)$ changes based on the flux above and below it using a continuity equation of the form
$$\pdiff{c}{t}(x,t) = - \pdiff{J}{x}(x,t).$$
The continuity equation captures the fact that the concentration only changes due to the movement of molecules determined by the flux $J$. Rather than derive this equation, your task is to make an argument why the form of the continuity equation makes sense.
Include the following pieces in your argument.
- What does the partial derivative $\pdiff{J}{x}(x,t)$ mean (in terms of how $J$ changes as one of the variables changes)?
- One special case of the equation is when $\pdiff{J}{x}(x,t)=0$. Justify that it makes sense that $\pdiff{c}{t}(x,t)$ would be zero in this case. Some steps could include:
- If $\pdiff{J}{x}=0$, what does that mean about the flux $J(x,t)$? (In particular, what does that imply about the flux just above and just below a point $x$, as in the above illustration.)
- If $\pdiff{c}{t} = 0$, what does that mean about the concentration $c(x,t)$?
- If the flux $J(x,t)$ is of that form, why should that imply that the concentration won't change?
- Imagine that $\pdiff{J}{x}(x,t) > 0$ at some point $x$. What does that mean about the flux just above and just below that point? Why would that form of the flux imply that the concentration $c(x,t)$ at $x$ should decrease?
- Imagine that $\pdiff{J}{x}(x,t) < 0$ at some point $x$. What does that mean about the flux just above and just below that point? Why would that form of the flux imply that the concentration $c(x,t)$ at $x$ should increase?
The continuity equation is a general equation that includes cases beyond diffusion, depending on the form for the flux $J(x,t)$. When we account for the manner in which the molecules diffuse, we will obtain a form of the flux $J(x,t)$ that will turn the continuity equation into the diffusion equation.
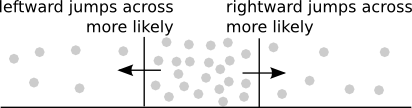
The above diagram illustrates an important feature of diffusion. Since the jumps of each molecule occur randomly either left or right, whether the flux across a point $x$ is more rightward or leftward depends only on whether there are more molecules immediately to the right or the left. The rightward arrow indicates where the flux would be positive as the higher concentration of molecules to the left will lead to more rightward jumps across that point. The leftward arrow indicates the reverse situation, where the negative flux of more leftward jumps is caused by the higher concentration to the right.
It turns out that, if the molecules are diffusing with diffusion coefficient $D$, the actual form of the flux will depend on the derivative of $c(x,t)$ with respect to $x$ according to the equation
$$J(x,t) = -D\pdiff{c}{x}(x,t).$$
Make an argument why this form of the flux makes sense.
Include the following pieces in your argument.
- One special case is when $\pdiff{c}{x}=0$. Justify that it makes sense that the flux $J(x,t)$ should be zero in this case. (Recall that $J(x,t)$ is the net rate of molecules jumping rightward across $x$. When $J(x,t)=0$, does that mean molecules are not jumping rightward? Rather, what does it mean about the rightward and leftward jumps? Why should that be true when $\pdiff{c}{x}=0$?)
- When $\pdiff{c}{x}(x,t) > 0$, what does this mean about the concentration $c(x,t)$. In that case, in which direction would you expect the net flux to be? Does this agree with the equation for $J(x,t)$?
- When $\pdiff{c}{x}(x,t)< 0$, what does this mean about the concentration $c(x,t)$. In that case, in which direction would you expect the net flux to be? Does this agree with the equation for $J(x,t)$?
The conclusion of this argument should be that the equation for $J(x,t)$ captures the fact that, with diffusion, molecules tend move from regions of higher concentration to regions of lower concentration.